End-of-life - simulation of reprocessing cycles
Maximum life-cycle testing of reusable medical devices
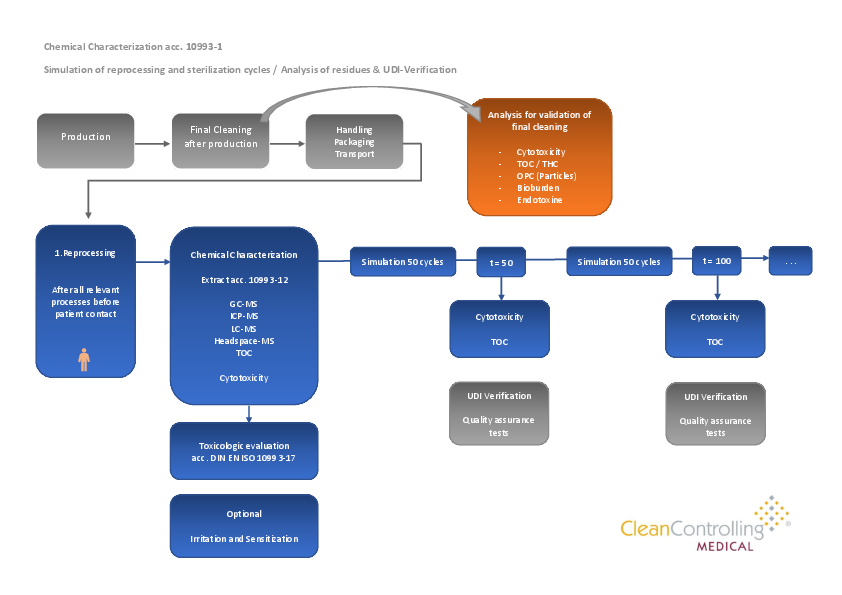
Reusable medical devices must also be tested with regard to biological safety during their lifetime in the sense of biocompatibility assessment according to DIN EN ISO 10993-1. The maximum lifetime of reusable medical devices is defined by the maximum number of validated reprocessing cycles. Lifecycle testing is required to assess biological safety during the lifetime.
Following the biocompatibility assessment with the chemical characterization and toxicological evaluation, the medical device is subjected to a simulation of several reprocessing cycles according to the validated manufacturer's instructions. After a defined number of reprocessing cycles, the biological safety of the product is evaluated, for example, by means of cytotoxicity and TOC. In addition, UDI verification and functional QA testing by the manufacturer also play an essential role here. Through several cycle series, the maximum lifetime of the products is determined and defined.
Procedure of the test
| Reprocessing | Cycle series typically with mechanical (RDG) reprocessing according to validated manufacturer specifications | ||
|---|---|---|---|
| Sterilization | Cycle series Steam sterilization according to validated manufacturer's specifications | ||
| Biological residue analysis | e.g. in-vitro cytotoxicity test according to DIN EN ISO 10993-15 | ||
| Chemicalresidue analysis | e.g. TOC determination (e.g. of tensides) |
Further Information
These tests are part of our accreditation, detailed information on the scope of accreditation can be found here.
You can also find detailed information on test procedures and test standards in our Infothek
If you have any questions, the employees from our sales team will be happy to help you.
Newsletter registration