The life cycle test – definition, procedure, and tests
Definition of the life cycle test
The life cycle test, also known as end-of-life simulation, is a systematic test that simulates the stress on a medical device over its entire service life. The aim is to verify the functionality, safety, and material resistance under realistic conditions of use. All relevant standards, such as ISO 10993 and ISO 17664, as well as the requirements of the MDR, are taken into account.
Procedures and testing strategies
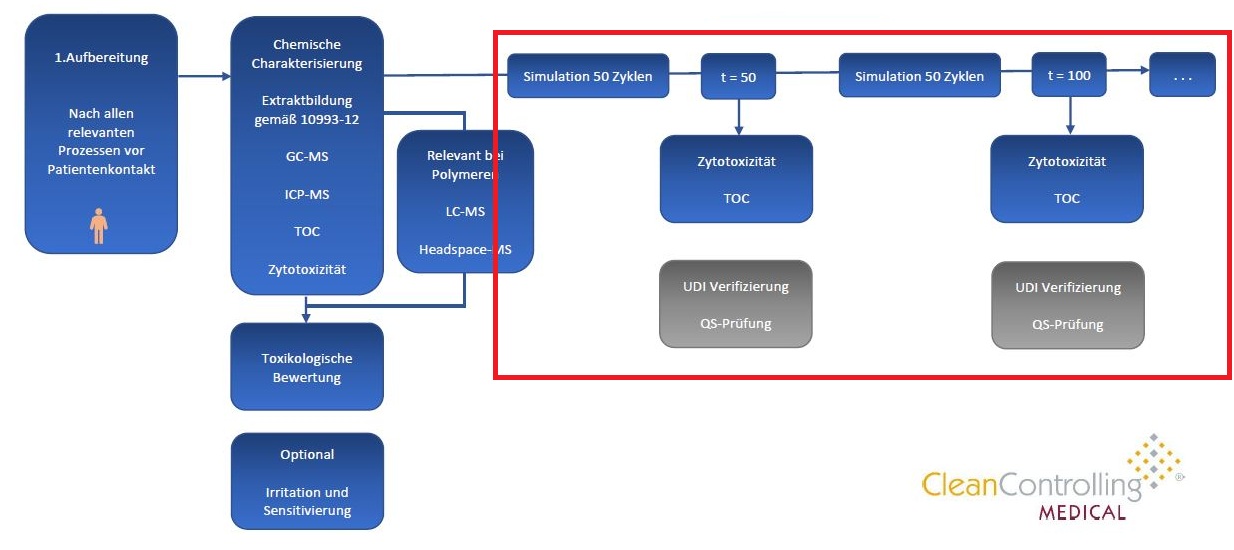
The life cycle test is carried out in several steps. First, repeated cleaning and sterilization cycles, as they occur in clinical practice, are simulated. Validated procedures such as machine cleaning in washer-disinfectors (WDs) and steam sterilization at 134 °C are used. Typically, up to 200 cycles are performed to determine the maximum service life. Between cycles, tests such as cytotoxicity tests and TOC analyses are performed to verify biological safety. In addition, functional tests and material analyses are performed to ensure the integrity of the product.
Relevant tests within the scope of approval
Various tests are required as part of the approval process. These include validating the service life, ensuring sterility and cleanliness after repeated use, and testing material resistance. All results are documented and serve as evidence for the regulatory authorities. These tests are essential to ensure compliance with the MDR and the relevant ISO standards.
Testing services from CleanControlling Medical
CleanControlling Medical offers customized testing concepts that are individually tailored to the respective product specifications and regulatory requirements. Using state-of-the-art reprocessing technology, we simulate the reprocessing process (without contamination). Thanks to our expertise and infrastructure, we can perform all necessary tests reliably and in accordance with standards.
Conclusion
Life cycle testing is an indispensable part of the approval process for medical devices. It ensures that products remain safe and functional throughout their entire service life. CleanControlling Medical's testing services provide manufacturers with a reliable basis for meeting all regulatory requirements and successfully launching their products on the market.
Newsletter registration